
A New Treatment Approach in the Arsenal: Neoadjuvent Therapy
Community is an essential ingredient to a successful revolution and is particularly critical when fighting advanced melanoma.
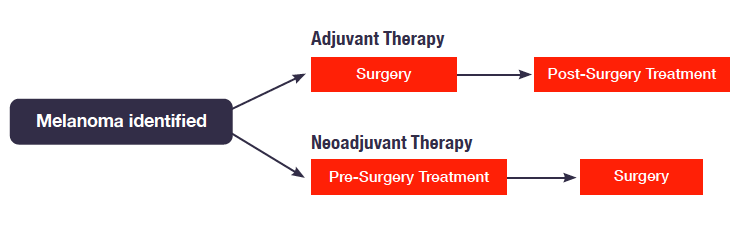
Surgery is the backbone of melanoma treatment and is curative for the vast majority of patients with localized melanoma. However, even if surgery successfully removes all detectible traces of tumor tissue, some patients will still experience a melanoma relapse.
That’s because, in some patients, residual cancer cells remain even
though they can’t be found by blood draws, scans, or other tests. These
microscopic melanoma cells lurk, hidden in the body, and wait. It is like going to war with an invisible enemy who is playing hide and seek. Harder still, doctors and researchers don’t know which patients this enemy may be hiding in until it comes back.
This is why knowledge sharing among allies is paramount. Revolutions can’t be won without strong partnerships. This is why in its quest to end suffering and death due to melanoma, MRA has been steadfast in its commitment to bring all stakeholders to the table to ask tough questions and to share lessons learned broadly.
“There’s the dangerous tendency, not just in the melanoma field but in the cancer field broadly, for ‘camps’ to form among researchers. Camps quickly become divisive and in my view are an intolerable state in the field,” warns Dr. Keith T. Flaherty, Director for Targeted Therapy and Clinical Research at Massachusetts General Hospital and a Professor of Medicine at Harvard Medical School.
Flaherty says this is why the convening work that MRA does is so critical. “You do not want people trying to divide up cancer biology into distinct domains and losing focus on the reality that there is effectively cross talk between every aspect, every molecular aspect and feature of cancer with every other,” says Flaherty. “They work in concert to create the problem and then similarly on the therapeutic side we have to unravel the problem by maintaining that same mindset.”
What the field is seeing now is that the same therapies that are having an impact in advanced
melanoma are having a comparable impact in eradicating microscopic deposits of residual disease. Flaherty points to neoadjuvant therapy with BRAF inhibitors and immune checkpoint therapy and to MRA for helping shift the focus towards this promising approach.
Neoadjuvant therapy is a presurgical approach. Patients with clinically detectable stage III melanoma, for example, may seek neoadjuvant therapy because their melanoma has signs that indicate a higher risk of recurrence, such as lymph node involvement or melanoma lesions deeper than 4mm. Early results from clinical trials indicate that this approach decreases the risk of recurrence of melanoma in patients. To help explore this groundbreaking area, the MRA co-hosted a public workshop with the Food and Drug Administration (FDA) on November 6, 2019. The workshop focused on identifying, discussing, and addressing key issues, challenges, and opportunities in pursuit of neoadjuvant therapies for patients with surgically resectable melanoma.
MRA’s Neoadjuvant Therapy Funding
Beyond the MRA-FDA co-sponsored meetings, MRA is helping advance neoadjuvant research through two large Team Science Awards:
- Overcoming upfront resistance to neoadjuvant CTLA-4 plus PD-1 blockade
MRA Team Science Award with Young Investigator supported by Amanda and
Jonathan Eilian Christian Blank, MD, PhD, Netherlands Cancer Institute - Predictors of response to neoadjuvant therapy in melanoma
RTFCCR-MRA Team Science Award Rodabe Amaria, MD, University of Texas MD Anderson Cancer Center
Treatment Paths for Adjuvant Versus Neo-Adjuvant Therapy
“One of MRA’s key roles is bringing all the stakeholders together and it’s really part of our mission to collaborate with everyone to advance the field of melanoma,” says Dr. Marc Hurlbert, Chief Science Officer at MRA. “Having a topic that was important to melanoma science and the regulatory agency was critical. The fact that we had this joint area that we wanted to work on and then to hold this public workshop where we could have broader dialogue was great,” he adds.
The planning for and involvement in the meeting involved a vast—and multidisciplinary—group: the FDA, MRA, volunteer advisors, and MRA’s Scientific Advisory Panel. It tapped experts from other cancer types including those who study breast and lung cancer specifically.
The public workshop brought together pioneers in neoadjuvant therapy as well as clinicians and physicians new to this therapeutic approach. Attendees included oncologists, surgeons, patients, epidemiologists, biotech, pharmaceutical companies, caregivers, and advocates. In addition to the FDA, members from the National Cancer Institute (NCI) and National Institutes of Health (NIH) were also present.
The FDA-MRA workshop coalesced into three thought-provoking discussion panels with an opportunity for discussion amongst speakers and audience members alike. The workshop sessions were recorded and are now available online. Next up is a peer-reviewed journal article. “Now the goal is to get this information out to thousands of clinicians and clinical trialists all over the world,” says Hurlbert.
Flaherty adds,
“The FDA will never tell the cancer field how to operate differently. Drug companies will never tell the cancer field how to operate differently in terms of how we think about the conduct of clinical trials, who we should be targeting in those clinical trials. Yes, we academics pose those questions
or make those statements, but we can’t make them individually. That’s not effective. We need to be able to come together. There’ll be inherent disagreements in those areas, so we need to work through those and find the areas of common ground or consensus. That’s exactly what the MRA has done. They’re helping us to understand what the barriers to its adoption in clinical trials are and, ultimately, in clinical practice.”
The more we can understand this, the more we can help patients. And the more we help patients, the more we can drive this revolution forward.

“To bring together the stakeholders and essentially create a blank slate in terms of agenda and who’s trying to accomplish what, for what purpose, that is what a group like MRA can uniquely do and they are the ones who’ve uniquely done it.”
Dr. Keith T. Flaherty